Research
Position:
English
>
Research
1. Proton Exchange Membrane Fuel cells (PEMFCs)/ Electrocatalysis
Polymer electrolyte fuel cells (PEFCs), one of the most promising energy conversion technologies available today, have major advantages over gasoline combustion, including better overall fuel efficiency and reduction in CO2 and other emissions. A major limiting factor of energy-conversion efficiency for present fuel cells is the sluggish kinetics of the oxygen reduction reaction (ORR) at the cathode. Platinum and its alloys are the most active ORR catalysts. But they suffer from high cost and low abundance. Besides, the long-term stability of the catalysts still need to be improved.
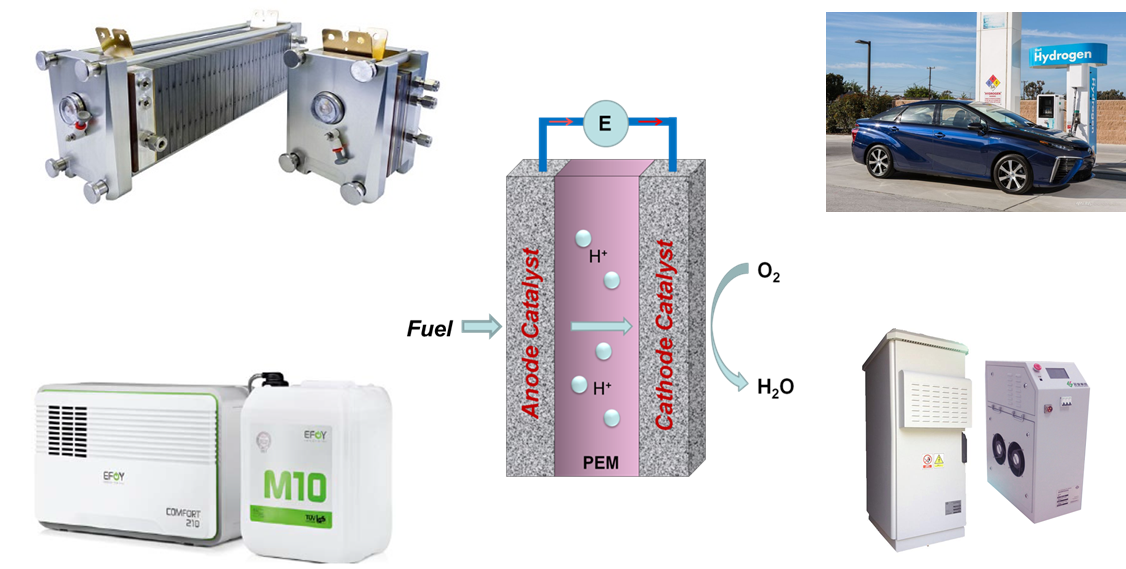
Electrocatalysis is a specific form of catalysis that function at electrode surfaces. The electrocatalyst assists in transferring electrons between the electrode and reactants, and/or facilitates an intermediate chemical transformation described by an overall half-reaction. It is a research hotspot for the energy conversion and storage technologies.
Representative Publications
Transition Metal-Nitrogen-Carbon (M-N-C) electrocatalysts for ORR
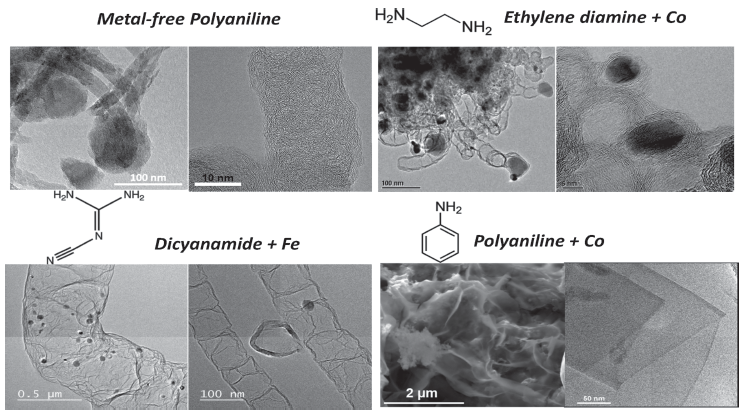
Transition Metal-Nitrogen-Carbon nanomaterials are a group of highly active ORR catalysts. In our work, we found that the nitrogen-containing precursor (polyaniline, ethylene diamine, Cyanamid, etc.) and transition metals (iron, cobalt, etc.) had great influence on the structure and catalytic activity of the final product (Adv. Energy Mater. , 2014, 4, 1301415.).
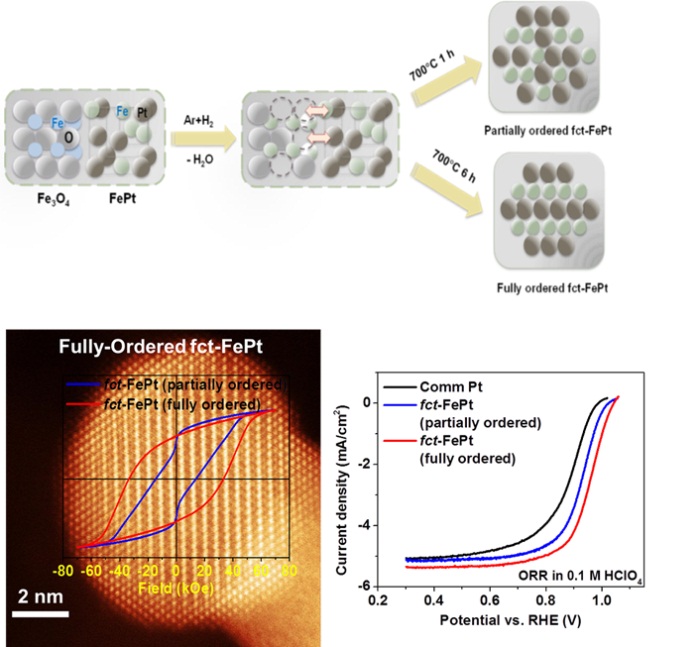
Fully-Ordered PtM (M = Co, Ni, Fe) Nanoparticles for ORR
The fully-ordered fct-FePt NPs is synthesized using dumbell FePt-Fe3O4 to introduce atomic defects during reduction annealing. They exhibit excellent activity (the most active fct-structured alloy NP catalyst ever reported) and stability (no Fe loss in acid). (Nano Lett. 2015, 15, 2468.)
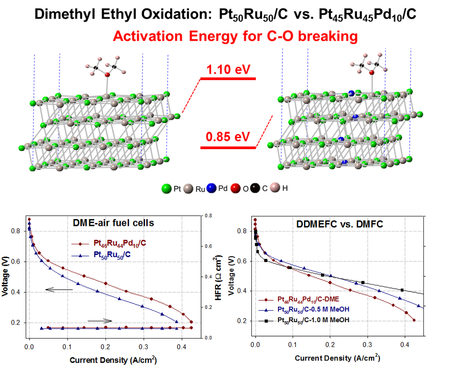
High-Activity PtRuPd/C Catalyst for Direct Dimethyl Ether Fuel Cell
Dimethyl ether (DME) has been considered as a promising alternative fuel for direct-feed fuel cells but lack of an efficient DME oxidation electrocatalyst has remained the challenge for the commercialization of the direct DME fuel cell. We designed a ternary carbon-supported PtRuPd catalyst for DME electrooxidation. As evidenced by both electrochemical measurements in an aqueous electrolyte and polymer-electrolyte fuel cell testing, the ternary catalyst shows much higher activity (two-fold enhancement at 0.5 V in fuel cells) than the state-of-the-art binary Pt50Ru50/C catalys. (Angew. Chem. Int. Ed., 2015, 127, 7634; US Patent: US20130292260 A1)
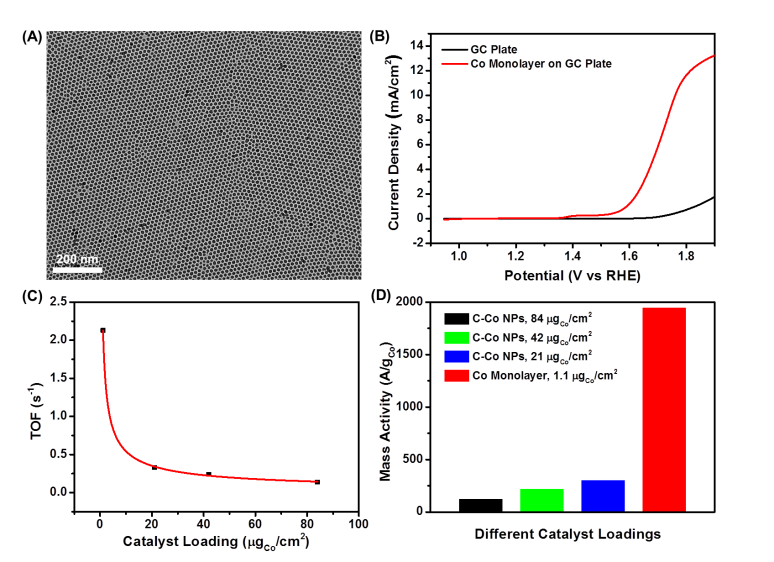
Monolayer Nanocrystals Assemblies for Oxygen Evolution Reaction
Monodisperse cobalt (Co) nanoparticles (NPs) were synthesized and stabilized against oxidation via reductive annealing at 600°C. The stable Co NPs are active for catalyzing the oxygen evolution reaction (OER) in 0.1 M KOH, producing a current density of 10 mA/cm2 at an overpotential of 0.39 V (1.62 V vs. RHE, no iR-correction). Their catalysis is superior to the commercial Ir catalyst in both activity and stability. These Co NPs are also assembled into a monolayer array on the working electrode, allowing the detailed study of their intrinsic OER activity.(J. Am. Chem. Soc., 2015,137, 7071.)
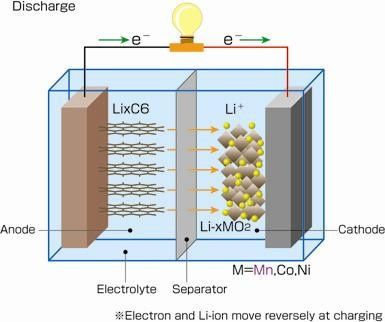
2. Lithium/Sodium Batteries
Lithium (sodium) batteries are a group of batteries that use Li (Na) or Li (Na) alloy as anode. Taking lithium ion battery for example, lithium ions move from the negative electrode to the positive electrode during discharge and back when charging.
Representative Publications
Transition Metal Oxides as Advanced Anodes for Lithium-ion Batteries
Transition metal oxides have recently received a great deal of attention as very promising anode materials due to their high theoretical capacity, good safety, eco-benignity, and huge abundance. Herein, we synthesized a series of transition metal oxides (Nano Energy, 2014, 6, 73; Nano Energy, 2014, 9, 334.). These materials demonstrated good electrochemical behaviors and might be used in the commercial lithium-ion batteries.
Transition Metal-Nitrogen-Carbon Nanomaterials for Li–O2 Batteries
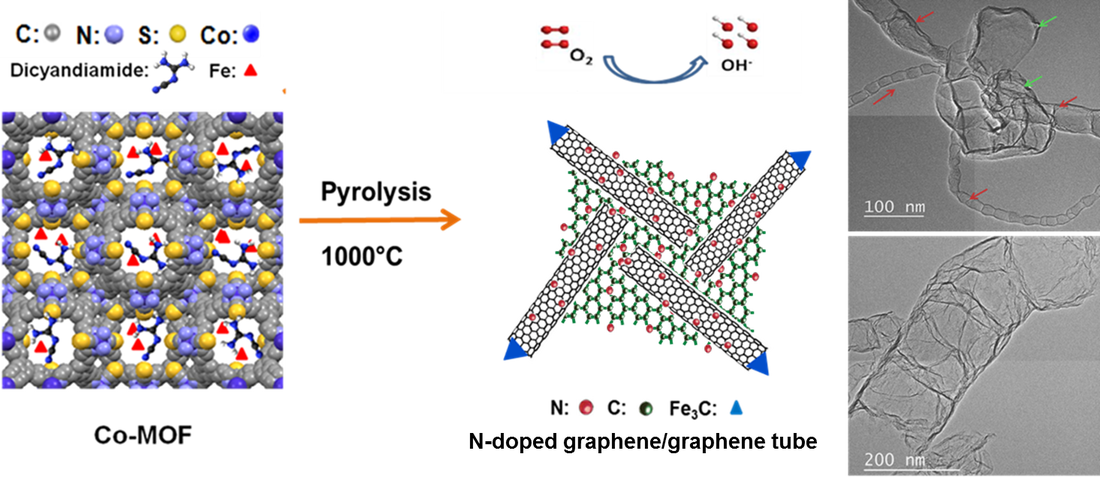
Nitrogen-doped graphene/graphene-tube nanocomposites (M-N-C catalyst) are prepared by a high-temperature approach using a newly designed cage-containing metal-organic framework (MOF) to template nitrogen/carbon (dicyandiamide) and iron precursors. The resulting N-Fe-MOF catalysts universally exhibit superior cathode performance in Li-O2 batteries. (Adv. Mater., 2014, 26, 1378)
3. Electroanalysis
Electroanalysis is a kind of technique in analytical chemistry which study an analyte by measuring the electrochemical signal (such as potential, current, conductance, charge) in an electrochemical cell containing the analyte. With the development of nanotechnology, electroanalytical methods now play more and more important roles in the field of life science.
Ion-Transfer Processes at Nanoscopic Liquid/Liquid Interfaces
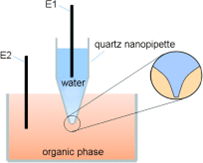
We fabricated quartz nanopipettes with radii of less than 5 nm for the first time. The fastest kinetic data for ion transfer (up to 140 cm?s?1) at a liquid/liquid interface were evaluated by nanopipette voltammetry. The steady-state voltammograms can be explained by theoretical models. (Angew. Chem. Int. Ed., 2009, 48, 8010.)
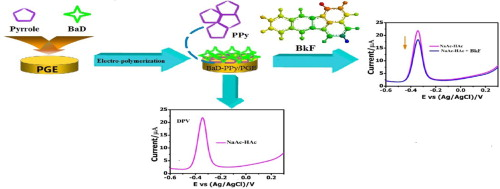
Electrochemical sensors
Nanomaterials such as Graphene and polypyrrole/benz[a]anthracene-7,12-dione were fabricated. These materials greatly enhanced the oxidation signals of various analytes, such as ascorbic acid (AA), dopamine (DA), uric acid (UA), xanthine (XA), hypoxanthine (HXA), bisphenol A (BPA), ponceau 4R, and sunset yellow. (Anal. Chim. Acta., 2014, 825, 26; Electrochim. Acta, 2014, 130, 187.)